ctx clinical trial
CTX - Clinical Trials Exemption. Providing High-Quality Most-Comprehensive Cutting-Edge Treatments.

Regulatory Requirements For Clinical Trials Australia Vs The Us
KANAGAWA Japan June 7 2022 PRNewswire -- Chordia Therapeutics Inc.
. You may be eligible to participate in a cerebrotendinous xanthomatosis ctx clinical trial. The CTN pathway is by far the most frequent regulatory pathway in. Clinical trials CX-2009-002 a Phase 2 multi-arm study is now enrolling patients with human epidermal growth factor receptor 2 HER2-non-amplified breast cancer.
See listed clinical studies related to the coronavirus disease COVID-19 ClinicalTrialsgov is a resource provided by the US. All studies both privately and government funded are listed on clinicaltrialsgov. The RESTORE study is a Phase 3 clinical trial looking at an investigational medication called chenodeoxycholic acid also called Chenodal or CDCA.
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995 including without limitation our plans and expectations to present clinical data from the ongoing CTX001 clinical trials during the EHA Virtual Congress expectations regarding the abstracts that will be made available on. Join the CTX Alliance. The investigational therapies explored in clinical trials are key to improved therapies among the whole CTX community.
CTX Clinical Trial Exemption An approval process. 3 para 2 and Commission Guideline 2012C 30203 Art. Clinical Trials Xpress CTX is an initiative of the University of Texas System established to provide an efficient and scalable centralized operating model for conducting multi-site clinical trials.
Chordia a biotech company engaged in the research and development of novel therapies for cancers today announced that it has presented the interim results from the Phase 1 clinical trial of CTX-712 a selective pan-CDC-like kinase CLK inhibitor discovered by Chordia at the 2022. Participants Researchers Sponsors NCT02638220 Study Page. A Phase 1 Dose Escalation and Cohort Expansion Study of the Safety and Efficacy of Allogeneic CRISPR-Cas9-Engineered T Cells CTX110 in.
In the RESTORE study doctors will look at markers of CTX in the urine to see if they are lowered when CDCA is used to treat CTX. Adult 16 years of age and older and Pediatric under 16 years of age. Design with a Quality Approach Enhance Patient.
Its time to do clinical research differently. In order to look for a study click on Home Search. Please refer to European Guidance 2008C 16802 Art.
The study will evaluate the safety and efficacy of autologous CRISPR-Cas9 Modified CD34 Human Hematopoietic Stem and Progenitor Cells hHSPCs using CTX001. A Phase 1 Dose Escalation and Cohort Expansion Study of the Safety and Efficacy of Allogeneic CRISPR-Cas9-Engineered T Cells CTX130 in. Users are reminded that phase 1 trials conducted solely in adults and which are not part of an agreed PIP are not public in the EU CTR.
Since the last publication of Guideline for the application of Clinical Trial Import Licence CTIL and Clinical Trial Exemption CTX 5th Edition in 2009 we have witnessed robust growth in clinical research industry with the aim to achieve at least 1000 clinical trials to generate GNI of RM5784 million by the year 2020 in Malaysia. When tested in human myeloid cell lines K562 and MV-4-11 CTX-712 showed a strong inhibitory effect on cell proliferation IC 50 015 and 0036 μM. Doctors will also look at the safety and potential side effects of CDCA treatment.
Under the CTA scheme as with CTX sponsors will. CRISPR Therapeutics and Vertex have launched two Phase 3 trials to assess the safety and effectiveness of CTX001 an experimental gene-editing cell therapy one in children with sickle cell disease SCD and another for those with transfusion-dependent beta thalassemia TDT. Interventional Clinical Trial Estimated Enrollment.
Listing a study does not mean it has been evaluated by the US. None Open Label Primary Purpose. The study has 2 groups.
CTX is a rare progressive disorder that can affect the brain spinal cord tendons eyes and arteries. Join our mailing list to receive information and news as we begin to gather and expand the CTX community. Ad Clinical Trials Of Today Giving Patients Hope For Tomorrow.
Explore 416022 research studies in all 50 states and in 220 countries. Arek Socha from Pixabay. None Open Label Primary Purpose.
Through diverse collaboration and unique approaches CTTI creates solutions that help you change how clinical trials are designed and run. We are inviting people with Cerebrotendinous Xanthomatosis CTX who may be interested. Vertex Pharmaceuticals and CRISPR Therapeutics have reported positive interim results from two Phase III clinical trials of investigational ex-vivo CRISPRCas9 gene-edited therapy CTX001.
National Library of Medicine. The Therapeutic Goods Administration TGA directly reviews the planned clinical trial and must give their approval for the clinical trial to go ahead. Travere Therapeutics is conducting a Phase 3 clinical trial to examine the safety and efficacy of Chenodal to treat CTX.
Learn more about our vision Transforming Trials 2030 or find a CTTI solution to help you. Both Phase 3 studies will enroll up to 12 children ages 211. Build Better Faster Clinical Trials.
Interventional Clinical Trial Estimated Enrollment. Chenodal is not indicated for the treatment of CTX but has received a medical necessity determination in the US by the FDA. The CTX Alliance is a newly-formed patient organization solely dedicated to providing resources support and promoting research for CTX patients families and healthcare providers.
Praluzatamab ravtansine CX-2009 is a conditionally activated antibody-drug conjugate Probody therapeutic employing the DM-4 payload and targeting CD166 also known as. Interventional Clinical Trial Estimated Enrollment. Envisioned by the Texas Regional Clinical and Translational Science Award CTSA Consortium TRCC this collaboration brings together premier.
For more information about clinical trials being conducted by the National Institute of Health NIH Clinical Center in Bethesda MD contact the NIH Patient Recruitment. Clinical trials conducted in Australia are subject to various regulatory controls to ensure the safety of participants. Current CTX Clinical Trials.
The schemes previous name of CTX underscored the exemption given by the TGA to a sponsor from entering their therapeutic good in the ARTG before conducting a clinical trial. We have recently developed an orally available and highly potent CLK inhibitor CTX-712 and evaluated its anti-leukemic activities both in vitro and in vivo. 115 other CTX meanings.
This is a single-arm open-label multi-site single-dose Phase 123 study in subjects with severe sickle cell disease SCD. A Phase 123 Study of the Safety and Efficacy of a Single Dose of Autologous CRISPR-Cas9 Modified CD34 Human Hematopoietic Stem and. Log In Sign Up.
None Open Label Primary Purpose. CTX001 involves the engineering of a patients hematopoietic stem cells to generate high foetal haemoglobin levels. CTX - Cyber Threat XChange.

Tga Presentation Tga S Role In Clinical Trials Regulation And Admini

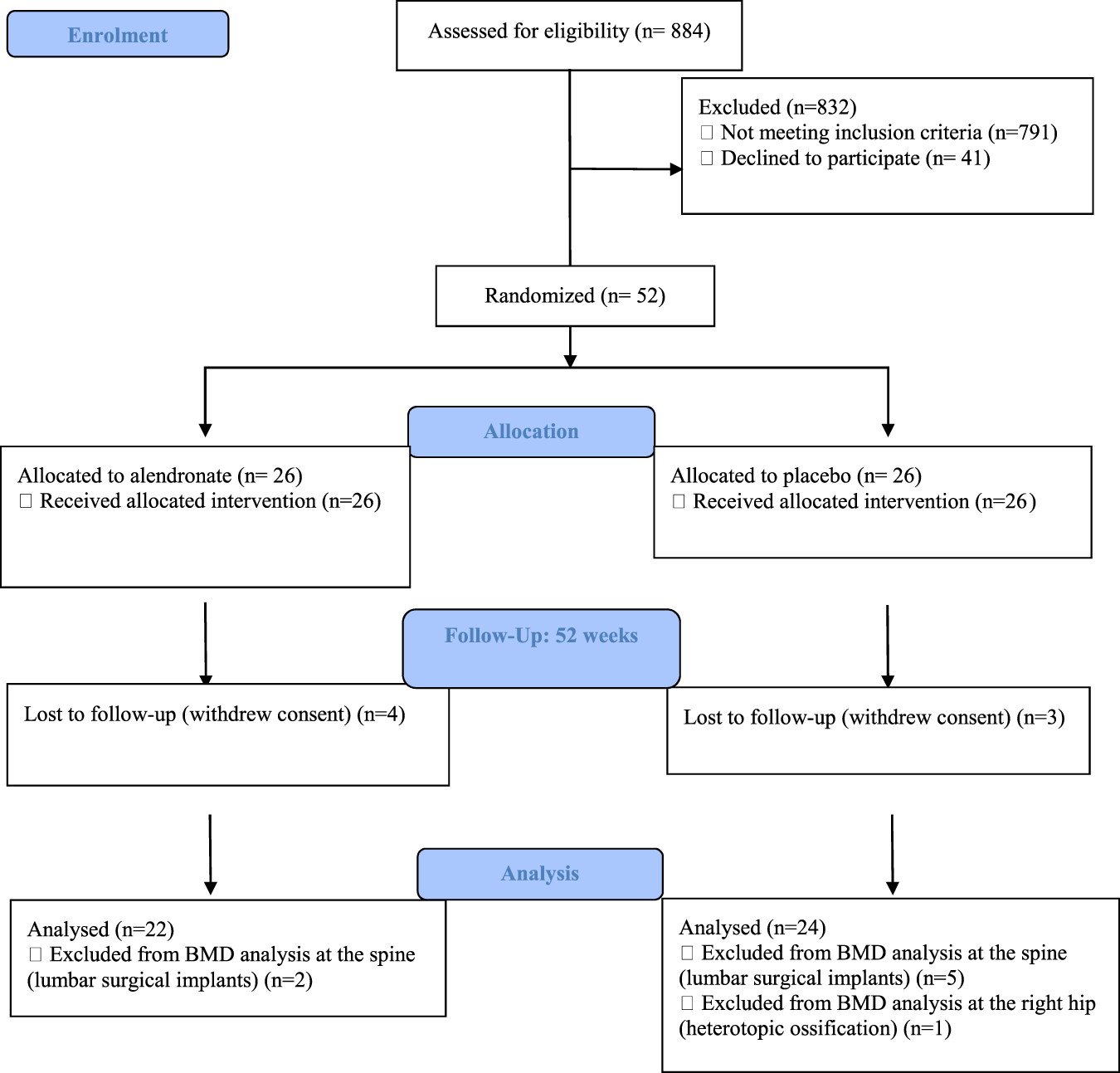
Preventive Treatment With Alendronate Of Loss Of Bone Mineral Density In Acute Traumatic Spinal Cord Injury Randomized Controlled Clinical Trial Spinal Cord
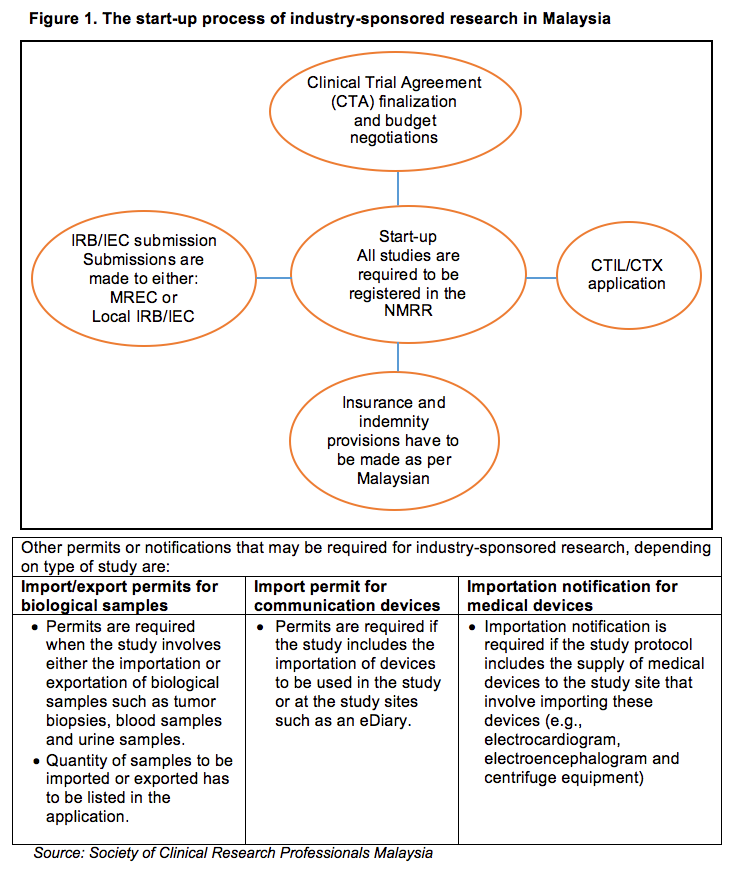
Malaysia S Clinical Research Ecosystem

Active Trials Head And Neck Cancer Alliance

Clinical Trials Medical Device Trials Genesis Research Services
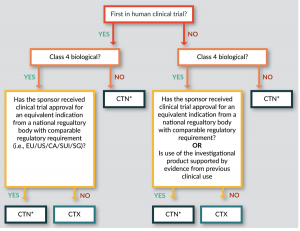
Bioinsights The Regulatory Environment For Cell Therapies In Australia An Opportunity To Expedite Clinical Development

Tga Presentation Tga S Role In Clinical Trials Regulation And Admini

Restore Study Cerebrotendinous Xanthomatosis Ctx Research Study
:%0A%0AcTBS%20followed%20by%20iTBS%20for%20Depression.png?md=1)
Gp2411 For Osteoporosis Clinical Trial 2022 Power
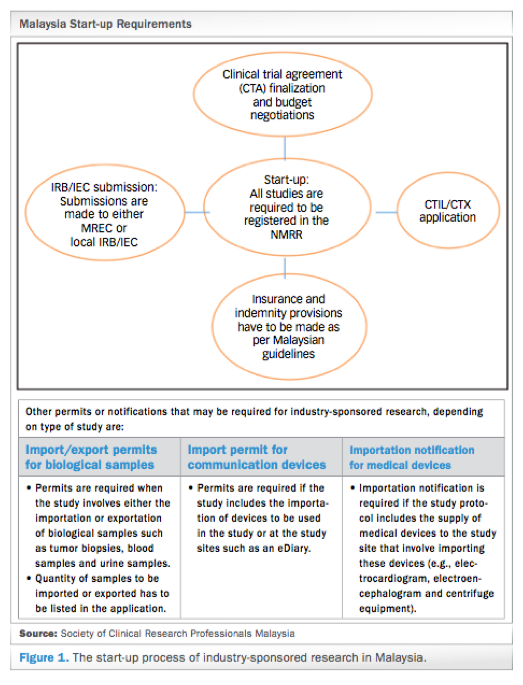
Malaysia S Clinical Research Ecosystem

Tga Presentation Tga S Role In Clinical Trials Regulation And Admini
Crispr Clinical Trials To Watch For In 2022 And Beyond

Development Milestones Development Milestones Clinical Trials Development
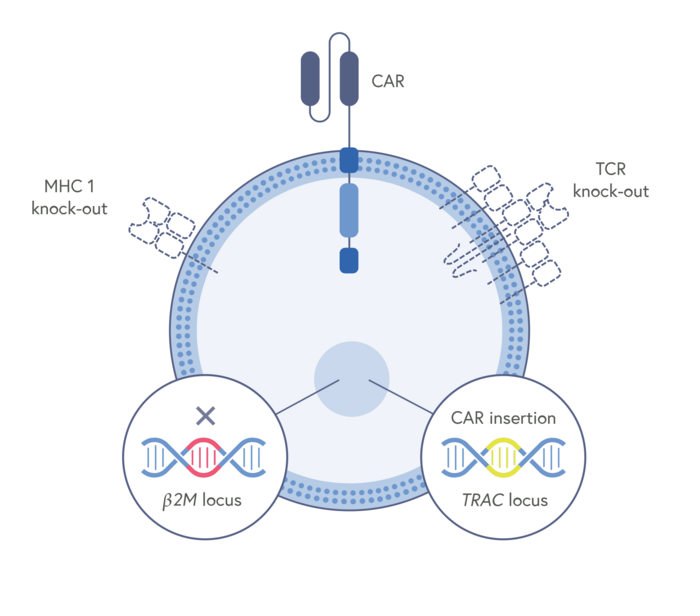
News Latest Gene Editing Clinical Trial Crispr Medicine

Ntx 1088 For Tumors Solid Clinical Trial 2022 Power
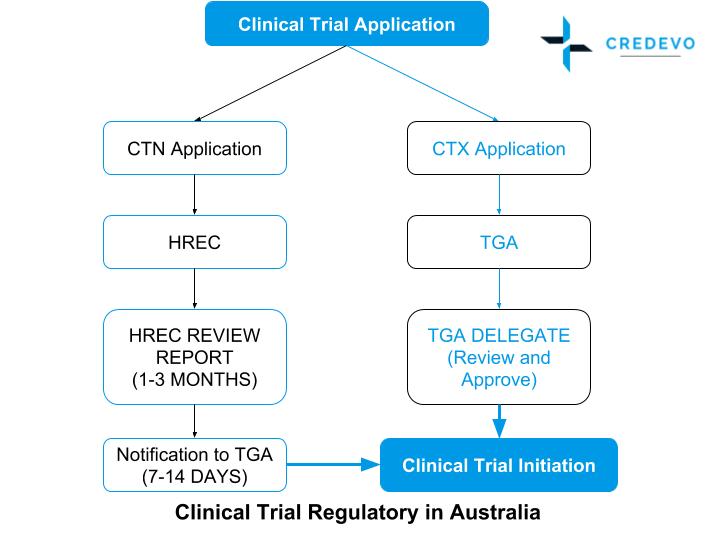
How To Get Started With Your Clinical Trials In Australia

Clinical Trial Application Cta In South Korea

Clinical Trials Medical Device Trials Genesis Research Services

Comments
Post a Comment